Nite Glow Powder
Nite Glow is our 15-minute glow-in-the-dark powder. It is whitish in shade with a familiar greenish glow. When we leave at the end of the day, it is our little box of Nite Glow which lights our way out the door.
- Ingredients
- Details
- Documents
Particle size (D50: 21 +/- 3 microns).
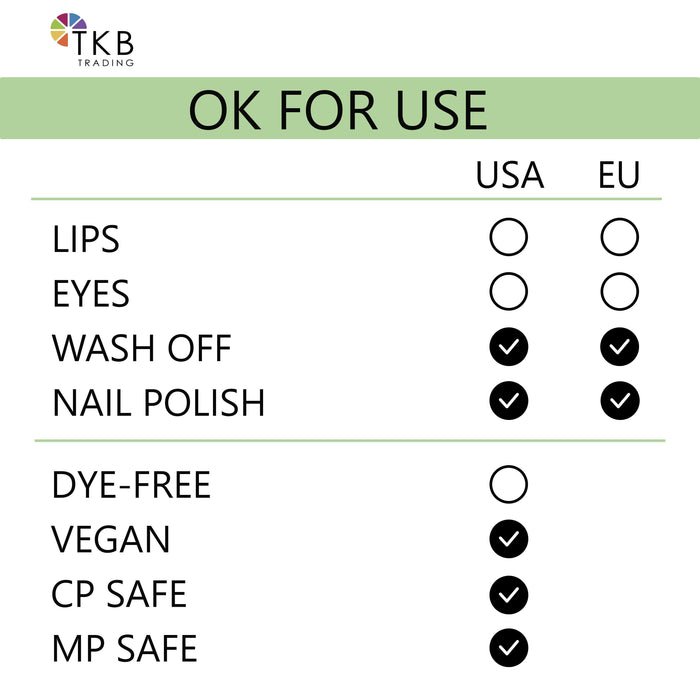
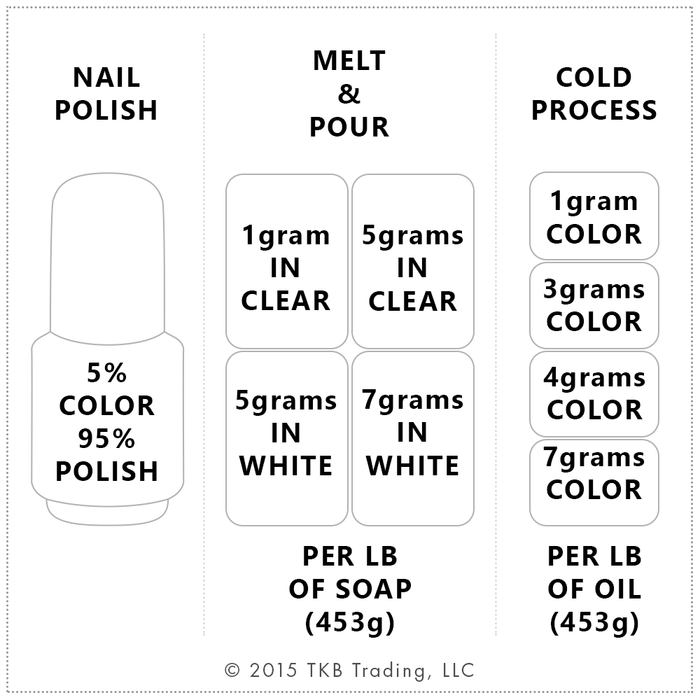
Nite Glow may be used for all kinds of interesting projects. In soap and gel candles, use it to color embeds. It can be added to nail polish, potpourri, face paint for novelty projects. You can also add it to translucent painting mediums and paint your craft projects.
In cosmetics, the FDA approves this product for limited use. Here is the specific FDA approval information on the product:
§73.2995 Luminescent zinc sulfide.
(a) Identity:
The color additive luminescent zinc sulfide is zinc sulfide containing a copper activator. Following excitation by daylight or a suitable artificial light, luminescent zinc sulfide produces a yellow-green phosphorescence with a maximum at 530 nanometers.
(b) Specifications:
Luminescent zinc sulfide shall conform to the following specifications and shall be free from impurities other than those named to the extent that such impurities may be avoided by good manufacturing practice:
- Zinc sulfide, not less than 99.8 percent.
- Copper, 100±5 parts per million.
- Lead, not more than 20 parts per million.
- Arsenic, not more than 3 parts per million.
- Mercury, not more than 1 part per million.
- Cadmium, not more than 15 parts per million.
(c) Uses and restrictions:
The color additive luminescent zinc sulfide may be safely used for coloring externally applied facial makeup preparations and nail polish included under §720.4(c)(7)(ix) and (c)(8)(v) of this chapter, respectively, to the following restrictions:
- (1) The amount of luminescent zinc sulfide in facial makeup preparations shall not exceed 10 percent by weight of the final product.
- (2) Facial makeup preparations containing luminescent zinc sulfide are intended for use only on limited, infrequent occasions, e.g., Halloween, and not for regular or daily use.
(d) Labeling requirements.
- (1) The label of the color additive and any mixtures prepared therefrom shall bear expiration dates for the sealed and open container (established through generally accepted stability testing methods), other information required by §70.25 of this chapter, and adequate directions to prepare a final product complying with the limitations prescribed in paragraph (c) of this section.
- (2) The label of a facial makeup preparation containing the color additive shall bear, in addition to other information required by the law, the following statement conspicuously displayed:
- Do NOT use in the area of the eye.
(e) Exemption from certification:
Certification of this color additive is not necessary for the protection of the public health, and therefore batches thereof are exempt from the certification requirements of section 721(c) of the act.
[65 FR 48377, Aug. 8, 2000; 65 FR 75158, Dec. 1, 2000]